MDCG and AIB release guidance on interplay between EU MDR/IVDR and AI Act

MDCG 2025-6 and AIB 2025-1, released on June 19, 2025, and setting out the interplay between EU MDR/IVDR and AI Act.
European Commission releases updated MDCG 2019-11 guidance on software qualification and classification under MDR and IVDR

EU updates MDCG 2019-11 guidance on qualification and classification of medical device software
MDCG 2025-04: New EU guidance on ensuring safe distribution of medical device software via online platforms

On June 16, 2025, the European Commission published the guideline MDCG 2025-4 outlining the requirements for the safe provision of software apps classified as medical devices on online platforms.
Team NB Publishes Press Release on Certificates with Conditions

On 12 March 2025, the TEAM NB issued a press release on certificates for which special conditions, regulations or even restrictions can be invoked in accordance with EU MDR 2017/745 and EU IVDR 2017/746 Annex VII Section 4.8.
ISO 15223-1 First Amendment Published
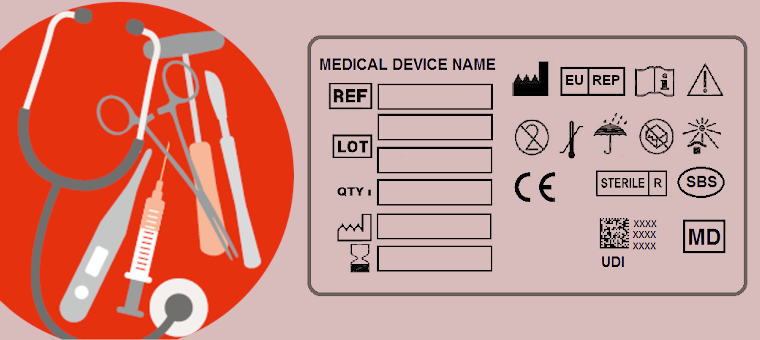
The harmonized standard ISO 15223-1 defines the symbols used by medical device manufacturers to label and communicate important information.
Update on the Medical Devices Operator Ordinance (MPBetreibV) and Medical Devices Dispensing Ordinance (MPAV)

Germany amends the Medical Devices Operator Ordinance (MPBetreibV) and Medical Devices Dispensing Ordinance (MPAV)
New Regulation (EU) 2025/40 on packaging and packaging waste

The EU adopts a new Regulation (EU) 2025/40 on packaging and packaging waste, which introduces stricter obligations focused on a circular economy.
MDCG guidance alert: New MDCG 2024-16 guidance on how to deal with interruption or discontinuation

New MDCG guidance provides an information form for MD and IVD manufacturers to complete in case of supply interruption or discontinuation.
MDCG 2022-5 Rev.1: Updated guidance on borderline products

Revised MDCG 2022-5 Rev.1 aims to improve classification of borderline products with new examples and explanations.
MDCG 2024-13: Regulatory status of ethylene oxide (EtO) intended for the sterilisation of medical devices

MDCG 2024-13: EtO is subject to the MDR/IVDR, although EtO as a substance does not require additional conformity assessment.


