NEWS
Medical Devices – Latest News
MDCG and AIB release guidance on interplay between EU MDR/IVDR and AI Act
MDCG 2025-6 and AIB 2025-1, released on June 19, 2025, and setting out...
Read MoreEuropean Commission releases updated MDCG 2019-11 guidance on software qualification and classification under MDR and IVDR
EU updates MDCG 2019-11 guidance on qualification and classification of medical device software
Read MoreMDCG 2025-04: New EU guidance on ensuring safe distribution of medical device software via online platforms
On June 16, 2025, the European Commission published the guideline MDCG 2025-4 outlining...
Read MoreTeam NB Publishes Press Release on Certificates with Conditions
On 12 March 2025, the TEAM NB issued a press release on certificates...
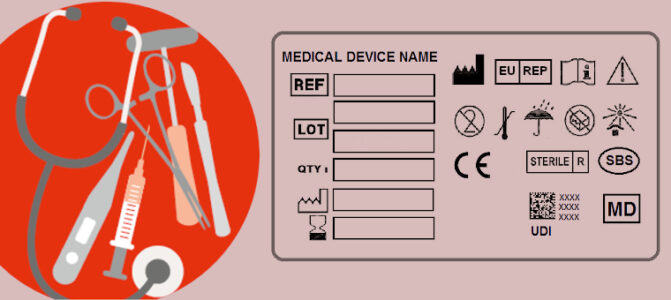
Read MoreISO 15223-1 First Amendment Published
The harmonized standard ISO 15223-1 defines the symbols used by medical device manufacturers...
Read MoreSubscribe to our News
Stay up to date on regulatory issues relating to the approval and marketing of medical devices