MEET us at MEDICA-COMPAMED 2023

Join SCC at MEDICA-COMPAMED in Dusseldorf on 13-16 November 2023
UK MDR: GB extends the acceptance of CE marked medical devices

MHRA announces the deadline extension for the acceptance of CE marked medical devices and IVDs shortly before the expiry of the transitional period.
Notified Body Confirmation Letter – EU 2023/607

Notified Body Confirmation Letter – EU 2023/607 6 June 2023 The European Commissioners have issued a new confirmation letter on the recent amendments to the Medical Device Regulation (MDR + IVDR). The eagerly awaited publication of the Template for NB – Confirmation letter in the framework of Regulation (EU) 2023/607 has now been officially communicated […]
MDCG 2020-3 Rev.1: Guidance on significant changes to transitional provisions under Article 120 MDR revised

MDCG 2020-3 Rev. 1: Guidance on significant changes to transitional provisions under Article 120 MDR revised 31 May 2023 Medical devices placed on the market after the effective date of the Medical Device Regulation (EU) 2017/745 (MDR), 26 May 2021, and compliant with MDCG Guidance 2021-25 are considered “legacy devices“. These include: “devices which are […]
Team-NB publishes best practice guidance for the submission of technical documentation for IVD MD
Team-NB publishes best practice guidance for the submission of technical documentation for IVD MD 3 May 2023 In February 2023, the European Association of Medical devices Notified Bodies, or Team NB, published the position paper „Best Practice Guidance for the Submission of Technical Documentation under Annex II and III of In Vitro Diagnostic Medical Devices […]
Team-NB revises the best practice guidance for the submission of technical documentation for medical devices
Team-NB revises the best practice guidance for the submission of technical documentation for medical devices 2 May 2023 On 19th April 2023, the European Association of Medical devices Notified Bodies, or Team-NB, published Version 2 of the position paper “Best Practice Guidance for the Submission of Technical Documentation under Annex II and III of Medical […]
gempex and SCC– partnership for medical device industry
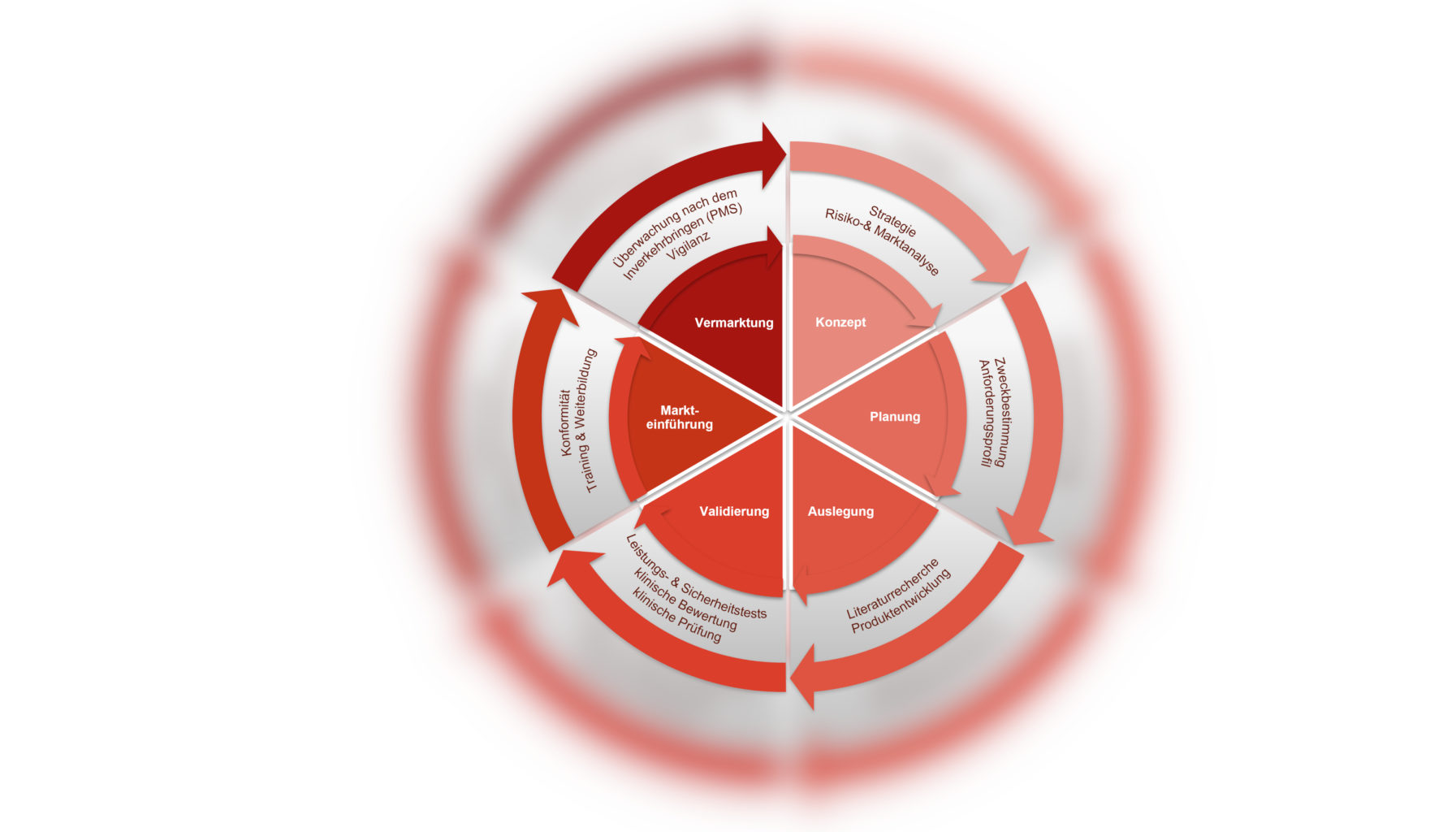
More than the sum of single services
European Commission extends MDR transition period

On 7 March 2023, the EU Council adopted the Commission’s proposal granting notified bodies and manufacturers more time to certify medical devices.
MDCG guidance on classification rules for IVDs revised
MDCG updates the guidance on classification rules for in vitro diagnostic medical devices
New MDCG guidance – questions and answers on vigilance terms and concepts according to MDR available

New MDCG guidance on vigilance terms and concepts according to MDR available since February 13, 2023


