EUDAMED: First four modules confirmed operational

On 27 November 2025, the EC published Decision (EU) 2025/2371, confirming the functionality of specific electronic systems within the European Database for Medical Devices (EUDAMED).
New ISO 10993-1:2025 Standard for Medical Device Biological Evaluation Released

New MD biocompatibility standard, ISO 10993-1:2025, helps manufacturers identify, assess, and manage biological risks associated with materials, design choices, and tissue contact during a device’s intended use.
Let’s meet at MEDICA-COMPAMED 2025

Let’s meet at MEDICA-COMPAMED 2025 in Dusseldorf from 17-20 November
Updated Manual on Borderline and Classification for Medical Devices Released
The fourth version of the ‘Manual on Borderline and Classification for Medical Devices under Regulations (EU) 2017/745 and (EU) 2017/746’ was published on 12 September 2025.
Future restrictions on PFAS and current regulatory activities from FDA and EU REACH

PFAS in medical devices, typically large-molecule materials known as fluoropolymers, garnered significant public attention due to concerns about their potential impact on human health and/or the environment.
MDCG and AIB release guidance on interplay between EU MDR/IVDR and AI Act

MDCG 2025-6 and AIB 2025-1, released on June 19, 2025, and setting out the interplay between EU MDR/IVDR and AI Act.
European Commission releases updated MDCG 2019-11 guidance on software qualification and classification under MDR and IVDR

EU updates MDCG 2019-11 guidance on qualification and classification of medical device software
MDCG 2025-04: New EU guidance on ensuring safe distribution of medical device software via online platforms

On June 16, 2025, the European Commission published the guideline MDCG 2025-4 outlining the requirements for the safe provision of software apps classified as medical devices on online platforms.
Team NB Publishes Press Release on Certificates with Conditions

On 12 March 2025, the TEAM NB issued a press release on certificates for which special conditions, regulations or even restrictions can be invoked in accordance with EU MDR 2017/745 and EU IVDR 2017/746 Annex VII Section 4.8.
ISO 15223-1 First Amendment Published
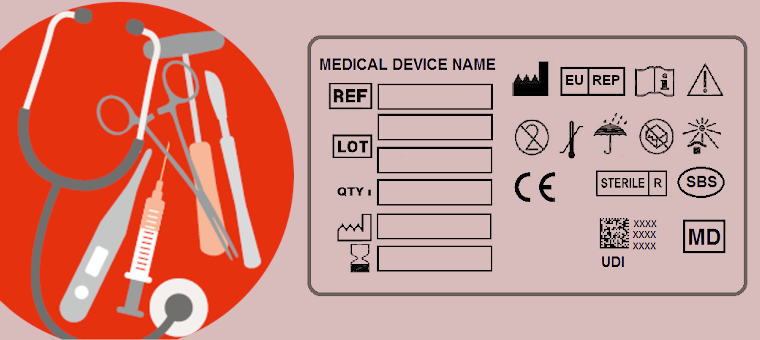
The harmonized standard ISO 15223-1 defines the symbols used by medical device manufacturers to label and communicate important information.


